Evolution of the Human Brain and the Myth of its Ten-Percent Use
Journal: Social Evolution & History. Volume 23, Number 2 / September 2024
DOI: https://doi.org/10.30884/seh/2024.02.02
Shahzadi Raheela Anum, Gomal Centre of Biochemistry and Biotechnology, Gomal University, D. I. Khan, KPK, Pakistan
Maria Shafiq, Medical Teaching Institution, Gomal Medical College, D. I. Khan, KPK, Pakistan
Sana Fatima, Gomal Centre of Biochemistry and Biotechnology, Gomal University, D. I. Khan, KPK, Pakistan
Abida Bibi, Gomal Centre of Biochemistry and Biotechnology, Gomal University, D. I. Khan, KPK, Pakistan
Amjad Ullah Khan, Gomal Centre of Biochemistry and Biotechnology, Gomal University, D. I. Khan, KPK, Pakistan
Shaista Naz, Gomal Centre of Biochemistry and Biotechnology, Gomal University, D. I. Khan, KPK, Pakistan
Hamna Batool Hashmi, Gomal Centre of Biochemistry and Biotechnology, Gomal University, D. I. Khan, KPK, Pakistan
Saqib Ali Rustam, Faculty of Veterinary and Animal Sciences, Gomal University, Dera Ismail Khan, KPK, Pakistan
Sobia Ishrat Khan, Government College for Women Rawalpindi, Punjab, Pakistan
Bushra Iqbal, School of Science, Harbin Institute of Technology, Shenzhen, China
Amna, Rawalpindi Women University, Rawalpindi, Punjab, Pakistan
Musavir Abbas, University of Science and Technology, Hefei, China
Muzammil Ahmad Khan, Gomal Centre of Biochemistry and Biotechnology, Gomal University, D. I. Khan, KPK, Pakistan
Jabbar Khan, Institute of Biological Sciences, Gomal University, Dera Ismail Khan, Pakistan;
Muhammad Muzammal*Gomal Centre of Biochemistry and Biotechnology, Gomal University, D. I. Khan, KPK, Pakistan;
*Corresponding author
ABSTRACT
The human brain is a complex organ that controls nearly every function of the human body. As science believes, humans evolved from apes but over time, humans achieved domain specification and maturation in the nervous system due to which they gained sensory, language, and other social cognition functions, called higher-level cognitive functions. Neurogenesis, or brain development, is an organized process that starts from the early weeks of pregnancy to early childhood. During development, brain parts connect to each other directly and indirectly to process information. As the brain is a mysterious organ so many theories and myths are linked to its development and its use. A widespread misconception, a claim, is that most people use only about 10 % of their potential brain power. But there is no room for this myth because we have now mapped almost every region of the brain. Furthermore, PET scans and fMRI clearly show that almost all parts of the brain are connected to each other and all parts are functionally active. And if the parts of the brain are unnecessary and unused, then they must be removed or disappear, according to the rule of the theory of evolution. Now it is the time to put the myth to rest even though it has survived for a whole century.
Keywords: myth, brain, evolution, 10% potential, apes.
1. INTRODUCTION
The human brain has been and continuous to be a source of mysteries and fascination for scientific communities (van Dijk and Lane 2020). The human brain controls and performs all the functions, from attention to individual behavior, due to its hierarchical development during childhood to adolescence (Baum et al. 2020). As mysteries are usually associated with myths, one such myth originated in the early 1900s and claimed that the average person uses only 10 % of their brain. Science is always about accepting and believing things with facts, in a similar way science has disproved this myth. There are different ways to trace the origin of this myth, but the most prominent statement was given by the pioneering American psychologist William James. He pointed out that the average person uses only a tiny fraction of their potential/brain, while most of it is unused and never developed. His statement about ‘tiny fraction’ slowly shifted to 10 % of brain's capacity; initially James stated that it was the use of 10 % of our gray matter for those who used it loosely. But over time, the myth was probably accepted by a few prominent figures of that time like Dale Carnegie, author of the famous book ‘How to Win Friends and Influence People’, German-born theoretical physicist Albert Einstein and American cultural anthropologist Margaret Mead, who have also been associated with 10 % brain use in the average person (Arora 2020). To discuss the 10 per cent brain myth, we are going to first discuss the human brain, its functions, and its functional connectivity.
2. RELIGION AND BRAIN DEVELOPMENT
Most of the time, religion is antiparallel to neuroscience in multiple theories. The theory of evolution first proposed an idea that humans gained their current appearance from existing animals such as apes or monkeys (https://www.nationalgeographic.com/science/article/when-darwin-met-another-ape). This idea was opposed by religious scholars (https://www.nationalgeographic.com/science/article/when-darwin-met-another-ape). But with the passage of time, the theory provided logical answers to the doubts that led to its acceptance by some people of different religions. The same is true of the theory of evolution, according to which humans evolved from apes. Initially, the theory of evolution was rejected by the world's major religions. The limited number of believers in both religion and science are in the circle of theistic evolution. According to theistic evolution, there is God who is the creator of everything, but every biological organism is the result of a natural process.
The evolution theory of human's evolution is first accepted by Buddhism (Awang et al. 2021). After that, the acceptance increased by multiple countries and religions, but the overall acceptance by people varied, such that the highest acceptance ratio was seen in the US and the EU, and the lowest acceptance was seen in the people of Turkey.
Islamic views on evolution are diverse. Islam is based on beliefs that humans were created by God and sent to the earth (Ibid.). But still, many Muslims believe that humans have evolved from existing life forms. But on the other hand, Muslims believe that life on earth started from a single point and that species were formed from clay. Thus, thinkers accepted the theory of evolution with the supremacy of God.
3. EVOLUTION OF THE HUMAN BRAIN
The brain is a central part of the human body. Understanding of the human brain is an answer to the question of how we evolved (Bae et al. 2015; Enard 2016). The human brain is an extraordinary organ that can perform distinctive functions and due to these characteristics, we can easily be distinguished from the great apes of Africa (our closest relatives) (Franchini and Pollard 2015; Funk and Gazzaniga 2009). Even though we have explored almost all human brains but the knowledge about our lineage is still incomplete (Bozek et al. 2014; Hrvoj-Mihic et al. 2013; Muraskin et al. 2016; Sherwood et al. 2012). Some cognitive abilities that are part of human behavior are also found in some rudiments like in apes. As the great apes have a more complex repertoire of natural behaviors than previously thought, and they transmit behavioral skills to their next generations in a manner similar to how our newborns learn by observing us (Kaminski et al. 2008; Premack 2007; Watson et al. 2017). In simple terms, we have evolved our mental abilities from our ancestors, and the major factor contributing to the adaptation and transmission of these behavioral skills is our genomic information. A number of studies are conducted to understand the development and evolution of the human brain from its ancestors. There are the following steps through which humans have evolved their mental abilities from apes.
3.1. Nervous System Expansion
The first ever step in the development of the human brain from the apes was the expansion of neurons into multiple centers. The early evolution of animals suggests that their nervous systems were made up of autonomic and somatic nervous systems to process information and control body movements respectively. Both systems originated from a rostral ampulla. During the era of development, animals gained size with the passage of time; in similar manners the nervous system also grew in size and complexity. Due to this complexity, the brain developed organs to communicate with the environment and to adapt to the environmental conditions in order to survive. For example, the bilateral animals have symmetrical bodies, so they can move only along the axis of their body plans, but with the development of their sensory and motor nerves, they were able to move in different directions. These animals developed the sensory organs at the location of the front extremity to receive the stimuli. This led to the evolution of the head through the development of functional axons, neuraxis centers, telencephalons, and then the cerebral cortex (Bretas et al. 2020). The development of the human brain, and the enlargement of its parts and the interconnection of these parts with the help of large neuronal networks, has led to the development in animals of different abilities such as communication, problem-solving intelligence, and even the more advanced ability of language. This development of sensory-motor processing machinery in animals is a leading factor in the advancement and formation of symbolic systems in humans (Figure 1C) which is responsible for language (Cassirer 2021).
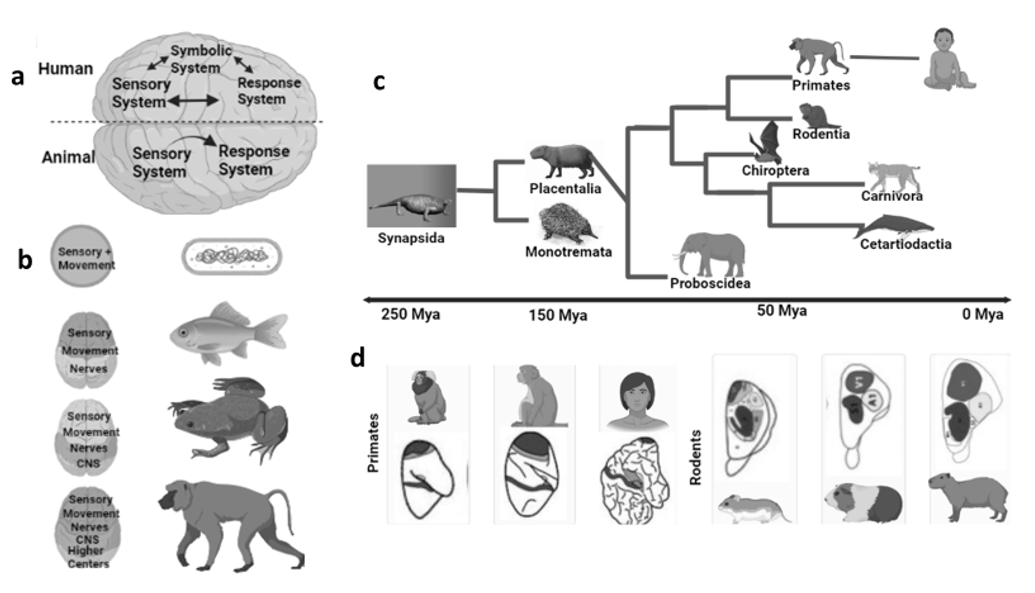
Fig. 1. Humans differ from animals because of their advanced
and extended sensory and motor nervous systems. a. A symbolic system
which is advanced in humans because of the connection of sensory neurons with motor neurons, which emerged as a single neuronal connection
in early protozoans. b. During evolution, the animal brain gains in weight
and complexity with the connection of bodily functions with the environment. While the complexity of the neural system
also achieved length increased because of the larger bodies of animals.
The continuous evolutionary process resulted in the grouping of neurons
for efficient speed of information processing and for the formation
of the central nervous system and eventually the telencephalon
and cerebral cortex. c. Phylogenetic tree of mammalian orders
diverged less than 100 million years ago. d. The organization of the brain
is different in different animals. Colored areas indicate
the sensory areas of the brain in early animals
Evolution is not only associated with anatomical changes, but also with the enlargement of the brain within species over multiple generations. One such example is the bats in a rural environment, which had brains expansion within a few decades due to adaptation to environmental changes (Snell-Rood and Wick 2013). Humans adapt to changes in habitat and ecology, according to their diet, geography, and population density (Enard 2016). All these factors are involved in the rapid selection of genes and shaping of humans in an environment such as tolerance to extreme temperatures, digestion of certain foods (lactose), or resistance to some diseases, etc. This kind of pressure is essential for development (Grabowski 2016; Laland et al. 2010). These are not only factors in brain development, but a rich diet is also involved (Grabowski 2016).
3.2. Coevolution of Gene and Culture
The second step of brain development is associated with genetic adaptation and gene selection through the process of natural selection. The ability of post-Neolithic humans to adapt to ecological conditions, made them linguistically and culturally rich. These abilities are considered a result of genomic adaptation, which emerged as a natural selection process to enhance behavioral flexibility (Crispo 2007; Roffet-Salque et al. 2018). The ultimate result of these adaptations was gene-culture (language) coevolution because this process allows a rapid colonization of new ecological niches and population expansion. A similar phenomenon was observed in killer whales, which exhibit vocal learning and consequent complex vocal communication (Foote et al. 2016). In the case of Homo sapiens (humans), our vocal tone is the result of the adaptation of two genes related to brain growth and development by natural selection process (Dediu and Ladd 2007; Evans et al. 2005; Mekel-Bobrov et al. 2005).
3.3. Thickness of Cortical Areas
The preadaptation mechanisms in animals lead to the development of vocalization and survival in harsh environments. However, it is still unclear at what stage the animals develop language-like features. However, after the successful expansion of the neuronal network and the development of language character, the animals got a distinct feature of improvement. The different existing characteristics start to improve in existing animals (Figure 1C), such as haptics in rodents, audition in bats, and vision in primates (Estrada et al. 2017). According to researchers the reason behind these improvements was the pressure of ecology for their survival (Kaas 1997; Petrides 1999). This pressure was likely to link with the enlargement of the cortical areas in the brain in primates. That was the reason why the brain size of these mammals, rodents, and other primates resulted in larger brains than their ancestors (Figure 1D) (Ventura-Antunes et al. 2013). But if we talk about cortical expansion, it will be due to the expansion of sensory and motor neuronal networks in the early transitions (Herculano-Houzel 2009). Thus, with the demand of ecological conditions, the brains start to add proportions to the brains along with novel features.
3.4. Triadic Niche Construction
Three spatial structures (triadic niche construction) act as mechanisms for such phenomena: (1) neural (brain), (2) cognitive, and (3) environmental niches that facilitate positive feedback loops (Figure 2). That is, human evolution has been characterized by a significant expansion of the brain and the consequent proliferation of newly functionalized regions, as well as with the continuous addition of new ways of knowing, such as the production and use of tools and language skills (Iriki and Taoka 2012). This expanded brain capacity creates rapid and drastic changes in human ecology that require more brain resources to accommodate them. Thus, in this way, the mechanism of evolution has shifted from a passive ‘natural selection’ to a positive triadic niche construction. Their continuous involvement in human life led to innovations and advances in the brain.
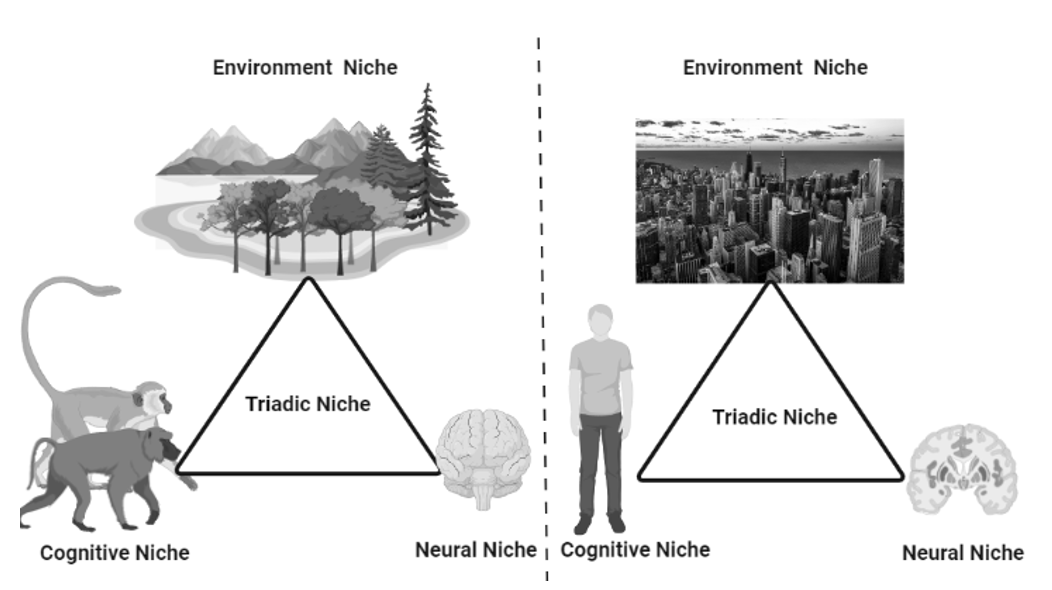
Fig. 2. The figure illustrates the adaptation of new cognitive abilities and brain expansion due to a continuous process called triadic niche
construction. It is the process and reason for an advanced form
of animal, the humans. In early apes, a loop had evolved between
environment, cognitive and neuronal niches resulting in a positive
adaptation in animals and a continuous expansion of functions.
The triadic interaction in ancestral primates (left) had expanded
through triadic niche construction to form modern humans (right)
4. BRAIN DEVELOPMENT
The adult human brain has a surface area of 1,820 cm3 along with 2.7 mm cortical thickness and 1,359 cm3 of volume (Pakkenberg and Gundersen 1997). The human brain is estimated to consist of 100,000 million (100 billion) neurons and the cerebral cortex contains about 20 per cent of these neurons (Herculano-Houzel 2009). The whole human brain system has almost 150 trillion synapses, because a single cortical neuron can connect to the other 7,000 neurons with its synaptic connections. And a human myelinated nerve fiber can cover a distance of 93,206 miles (Pakkenberg et al. 2003). But we cannot expect this from a brain that is enclosed in a small skull, but gyrification is the process through which a huge part of the brain is enclosed in a small area. Gyrification, folding of the cortical surface, is a mechanism to maximize the number of cortical neurons and minimize the overall fiber length within the confined space within our skull (Zilles et al. 2013).
4.1. Neuronal Division and Migration
Neurogenesis, the generation of neurons, is a step-wise process or set of highly arranged cellular events. At first, the neocortex develops from the neural tube. Radial glial cells are one of the earliest developmental cells in the neuroepithelium that extend from the neural tube. The neural tube is located near the outer embryonic brain vesicle, while the neocortex develops at the rostral end (Sidman and Rakic 1973). And it takes five weeks of gestation for the neuronal tube to close. At the same time, the formation of primitive ventricular takes place and in the same duration, the central canal starts to trap the amniotic fluid (O’Rahilly and Müller 2005). As the neural tube closes, it triggers an increase in the fluid pressure in the ventricles, which marks the onset of rapid brain growth.
4.2. Early Proliferation
Early proliferation starts between 4 to 5 weeks, at this stage the interkinetic nuclear migration takes place. This is a four-stage process to divide the neuroepithelial cells symmetrically at the margins of the ventricle (Bystron et al. 2008). In the first stage, the nucleus of the cells starts to arrange itself at the abventricular site or base (Figure 3). In the second stage, the cells start to move towards the ventricular surface. In the third stage, they reach the apical surface for symmetric division into progenitor cells. In the final stage, the cells return back to their basal position. The early proliferation involves an increase in progenitor cells, enhancement of the thickness of the ventricular zone, and increased surface area of basal positions. According to some neurologists, the fetal meninges are critically involved in brain development (Sun and Hevner 2014).

Fig. 3. The figure shows early development and neurogenesis.
It is characterized by the migration of interkinetic nuclei,
it is a four-phase process in which neuroepithelial cells
divide symmetrically at the edge of the ventricle.
Neurogenesis is a process when progenitor cells transform
from symmetric cells to asymmetric cells. Pyramidal neurons
are generated from apical progenitor cells in the ventricular zone
and basal progenitor cells in the sub-centricular zone
4.3. Development of the Cortical Plate
Around the seventh week, neurons migrating radially from the ventral and subventricular regions begin to form the cortical plate. In its early stages, the cortex is divided into two layers, a thin superficial marginal zone and an underlying subcortical plate. The marginal zone contains cells that migrate tangentially; these cells prevent the migration of radially migrating pyramidal neurons to enable cortex formation from the inside out (Rakic and Zecevic 2003). The peripheral area will eventually develop into cortical layer 1 (Raybaud et al. 2013). The basal subplate contains interneurons and post-migration pyramidal neurons, which are briefly connected by afferent axons until the cortex is ready to receive them (Molnár et al. 1998).
4.4. Maturation of Cortical Neurons
In the duration of 9 to 12 weeks, the subplate starts to increase its thickness with a significant reduction in the cell density (Carney et al. 2007). In this stage, the cortex gains thickness too while the mature cortical neurons reach their final developmental stage. Before reaching the final position, these neurons have specific shapes at different developmental stages, such as the nascent neurons, which have cylindrical cell bodies connected by axons to lower plates and dendrites (Bystron et al. 2008). As neurons age, their cell bodies become larger as the gradient progresses. They are round and have dendrites perpendicular to the cortical surface, this is the reason why older neurons develop connections more rapidly than younger ones (Sidman and Rakic 1973).
4.5. Maximum Thickness of Subplate
At 22 weeks, the differentiation of the cortical plate begins; it is basically a laminar, areal, and cytological differentiation. After that, gyrification begins in the parieto-occipital and central sulcus around the 24th week. Within 25–27 weeks, the ventricular region is reduced to a single-cell thick ependymal layer, but the sub-ventricular region continues to expand as it becomes a primary source of cortical neurons (Takahashi et al. 2012; Zecevic et al. 2005). It is the final stage for the maximum thickness of the sub-plate. After attaining the maximum thickness, the subplate starts to attenuate. However, some residual lower plate neurons persist throughout life as interstitial neurons in white matter tissue (Bystron et al. 2008; Kostović et al. 2002).
4.6. Final Development of Base Layer
By the beginning of 28 weeks, layer 1 is fully developed, containing the neurons filled with branches of apical dendrites and internal tangential axons. Upon the completion of the migration of neurons, the radial glial disappear (astrocytes) from the subcortical layers (Misson et al. 1991). However, interneurons migrate until the last trimester, at which point pyramidal neurons stop migrating (Raybaud et al. 2013). The movement of these neurons is confined to the limbic and medial regions because from this location they can enter the cortical plate, to develop a local circuit with cortical pyramidal neurons. After that, the most important and critical thing is the maturation and elongation of axonal parts during 24–34 weeks of gestation, and all these processes take place in the developing white matter of the brain (Holland et al. 2015). This is the same period that is associated with the onset of myelination. In myelination, the intermediate zone is first transformed into white matter tissue. This period is associated with the onset of myelination, which first transforms the midbrain into white matter tissue. Not only this but at the same time, the lower plate thins and the intermediate zone turns into white matter tissue. Together, multiple interacting modes of cell migration shape the developing neocortex. The mammalian brain requires a variety of progenitor cells (depending on species and developmental stage) for proper radial and tangential development.
5. THE MYTH OF THE 10-PERCENT BRAIN
The myth is a genre of folklore that includes such narratives which play important roles in society. Describing a narrative as a myth can be quite controversial, as ‘myth’ is usually used to indicate that a story is not objectively true. Similarly, a myth has been created about the human brain, which is a bit controversial and hard to believe that humans use 10 per cent of their brain. It all started with the experiments of Pierre Flourens.
Around the nineteenth century, Pierre Flourens, a French neurophysiologist, conducted a series of ground-breaking experiments on animals such as pigeons, chickens, and frogs. In these experiments, he repeatedly removed larger pieces of brain tissues. The objective of the experiment was to observe the effects of removing brain tissue on the behavior of the animals. The results showed that removing some parts of from the brain of these animals did not affect their normal functioning. The results were clearly consistent and fair. Based on these results, he stated that we can remove certain parts of the human brain without affecting its normal mental functioning (Flourens 1842). This laid the foundation for a popular myth, the 10 per cent brain, but in actual sense, it was about the use of a very small part of our brain – related to the most cited figure of 10 per cent. After that, another researcher, Charles-Édouard Brown-Séquard (1876), wrote about the power of the human brain: ‘rarely someone so well developed, perhaps no one fully developed’ (Hickok 2014). With the passage of time, the myth has deepened its roots in the scientific community, moving from William James's human use of a small proportion of the brain to Dale Carnegie's use of 10 % of the brain. The lines may be catchy, but they are unacceptable. There are multiple reasons to reject this myth, which are discussed below.
5.1. Animals as Model for the Human Brain
The base for this myth, provided by Pierre Flourens, was wrong in part because the methods he used to assess mental abilities were crude and his animal subjects did not do a good job of simulating human brain function. The notion that most of the potential of our brain is untapped is now unanimously rejected by the neuroscience community. The main difference between the human brain and the animal brain is that the human brain has extraordinary cognitive abilities, which is the most important achievement of evolution, while the cognitive abilities of the animal brain are relatively low. In addition, the cerebral cortex of the human brain responsible for higher cognitive abilities is particularly large and accounts for more than 80 per cent of the total brain mass (Figure 4), while the cerebral cortex of animal brains is smaller than that of humans. It is an unrealistic approach to compare the human brain with the animal/bird brain through experiments, because as the neocortex develops it is shaped by numerous interacting modes of cell migration, and a proper radial and tangential development of the mammalian brain requires different types of progenitor cells depending on the species and stage of development. So, the animal brain is made up of different types of progenitor cells than the human brain (Budday, Steinmann, and Kuhl 2015). Even if the dissection of human brain takes place, it needs a mechanism, called surface buckling, to release residual stress, but it requires unrealistic stiffness ratios, which is not possible (Richman et al. 1975; Ronan et al. 2014). Simply put, it is impossible to cut any brain parts into human brains and then check human mental abilities through observations because of the high probability of the subject's death.
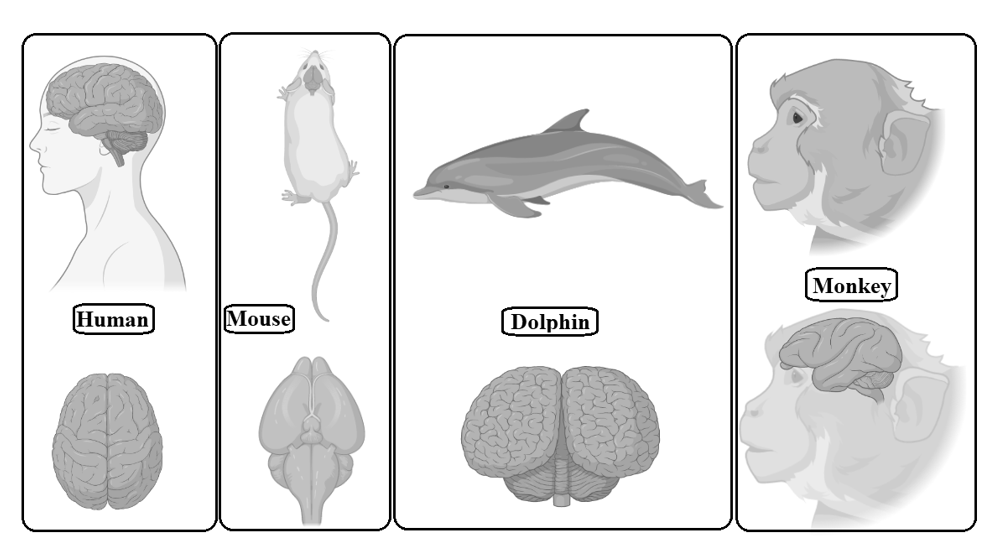
Fig. 4. Gross comparative neuroanatomy of different mammals
with the human brain. The human brain has an extremely wrinkled cerebral cortex compared to other mammals. This outer layer is responsible
for many functions, from information processing to language.
The human cerebral cortex contains billions of neurons
which are more than any other animal
5.2. Neurological Evidences
Neurological shreds of evidence prove that the 10-percent use of the brain is a myth, because there is not a single part whose absence does not affect the capabilities of humans, one such example is that the role of meninges is unknown, yet it causes disease if it is disrupted or injured (Sun and Hevner 2014). According to the myth, 10 per cent of brain is used while the rest is unused which means that removing or cutting a part of the 90 per cent will not affect the activities of the human body (Radford 1999). But this is not a thing that we can see by removing or cutting any part of the brain, because this can cause severe damage to human activities, such as a rare disorder called agenesis of Corpus callosum, in which the corpus callosum is partially or completely absent and is often associated with severe mental retardation (Palmer and Mowat 2014). The pieces of evidence do not stop here, as we know the unwanted or useless parts start to disappear. The same case can be seen in that after the neuronal migration is completed, the radial glial cells start to disappear from the subcortical layer or they can transform into astrocytes. This clearly proves that unnecessary cells and parts disappear (Misson et al. 1991). On the other hand, some neurons in the subplate persist throughout our lives because of their functioning in the grey matter; if they are not in use, they must have disappeared (Bystron et al. 2008). So, if we were on the ten- percent brain then the other 90 per cent must disappear.
5.3. Functional Connectivity of the Brain Pushes Back the Myth
In the final organization of the brain, when all components are fully developed, along with cell division and cell migration, the formation of connections begins and it is an important factor in the development of a normal and fully functional brain (Figure 5). By the second trimester, the cortex remains in an indirect connection with subcortical structures and underlying plates (subplate) rather than a direct connection with the CNS (Haynes et al. 2005). Once the connections start to develop, the exons also start to multiply their connections by extending their branches to other cortical and subcortical neurons. So, in the second half of pregnancy/gestation, neurons get involved in thousands of connections (Raybaud et al. 2013). In this phase, mechanical forces are strong and that is why they are studied extensively, because it is hypothesized by early researchers that surface morphogenesis is the result of these mechanical forces generated during compact wiring and axonal tension (a mechanism to bring the identical units closer to each other) during connectivity (Mitchison 1991; O’Toole et al. 2015; Suter and Miller 2011; Van Essen and Drury 1997). This hypothesis is still not fully understood because it can explain the gyrification, with realistic stiffness ratios, but it is not compatible with the physical experiment (Xu et al. 2010). According to some neurologists, buckling is the process that can reduce surface stress, but a physical experiment is still an unrealistic approach because the dissection is not complicated but a significant reduction in the stiffness ratio is not achievable (Richman et al. 1975; Ronan et al. 2014). The combination of these two mechanisms motivates the theory of stress – and strain-based growth, concepts consistent with realistic stiffness ratios and optimal principal stress distributions (Bayly et al. 2013; Budday, Nay et al. 2015).
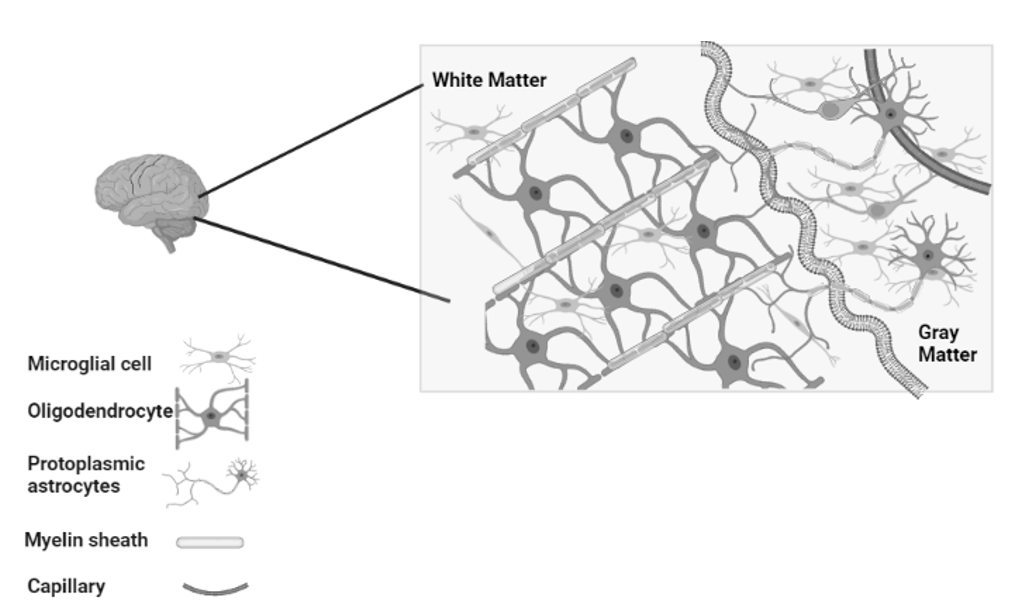
Fig. 5. An organized and fully developed brain. In the white matter,
myelinated axons allow rapid nerve impulse transmission;
Intermediate oligodendrocytes attach and form multiple myelin sheaths.
Fibrous astrocytes provide nourishment and synaptic processing.
In grey matter, neurons form synapses with each other
and with protoplasmic astrocytes. In both white and grey matter,
microglia help remove deposits and re-model synapses
In the human brain, the corpus callosum is one of the most distinctive connective structures and it is made up of millions (almost 20 million) of contralateral axons connecting the left and right cerebral hemispheres. These axons give a flat appearance under examination (Luders et al. 2010). Just like other neuronal bodies, the corpus callosum also differentiates as a commissural plate, an anatomical region in the human brain to provide an interhemispheric midline for telencephalic crossing, and this differentiation takes place around 8 weeks of gestation. In the human embryo, the exons of the corpus callosum develop in 12 weeks and mature at around 20 weeks (Achiron and Achiron 2001). The cortex begins to proliferate its neuronal layers for different connections to different parts of the brain. The cortex develops into layers in which the first layer of neurons or subplate neurons use to make connections with local and long-distance neurons, the second and third layers make connections with cortico-cortical fibers, interhemispheric and commissural plate, and intrahemispheric region, the fourth layer neurons connect with the them-cortical region, while the fifth layer makes the connection with the internal capsule, spinal cord, and brainstem. The last layer develops a connection with the basal grey matter, and the whole process of connectivity is completed in 17–32 weeks (Raybaud et al. 2013).
After 32 weeks, the final layering patterns of the neocortex appear with the development of short horizontal connections in the cortical gray matter and in the subcortical white matter. These connections are completed by 47 weeks of pregnancy (Marín-Padilla 2012). Not only neurons in layers but other cellular components also play an important role in the proper formation of neuronal connections, such as astrocytes, oligodendrocytes, microglia, and capillaries (Barres 2008). Indeed, radial glial cells in the lower plate and intermediate zone give rise not only to neurons but also to astrocytes and oligodendrocytes. The production and growth of these extra cells causes additional volume expansion in the corresponding layer (Mizutani et al. 2007).
5.4. A Marketing Trick by Influential
Another approach to this myth is that it can be a marketing strategy to sell research works, books, or even comics. Professor Anderson and Della Sala tried to find out the reality of this myth, but no one succeeded to find out the answer, even those working in the Einstein Archives could not find any record of it. It is open evidence that the 10-percent brain is a myth (Anderson and Della Sala 2012). In 1907 James published his article, in which he concluded that we use half of our mental capabilities and that we do not fully explore the fire that is caged in our bodies. He also stated that we use a small part of our mental and physical abilities. In the early days, the actual meaning was not fully understood by the people and due to such urgency the myth take hold but that does not mean that we only use 10 per cent of our brain.
According to Aamodt and Wang, it may be the nature of this 10-percent number that makes this myth so widespread as we say today, that first discovered it (Aamodt and Wang 2008). Dale Carnegie's marketing genius probably reinforced this idea by directly associating and putting numbers to the brain. Sandra Aamodt, author of Welcome to Your Brain, says this is why you lost your car keys, but your brain never forgot how to drive and other everyday problems. With this perspective, we can say that the human brain is always curious about the numbers around it, and that is why the myth of 10 per cent is most likely to be accepted and spread more than expected. A study carried out about ten years ago showed that half of Brazilians think they use only 10 per cent of their brain. These were the people who read Dale Carnegie's best-seller, which was translated into nearly every known language (Ibid.). On the other hand, it was considered that this myth could be a good way to motivate people and help them to improve themselves. People enjoy this pseudo fact because of its optimistic nature. This myth can make them feel that they have untapped potential that they are not using (Beyerstein 1987).
5.5. Surgeries are Proof of Full Use of the Brain
The 10-percent myth is not true and there is no room for this doubt – this has been proven by Functional magnetic resonance imaging (fMRI). This fMRI looked at all parts of the brain and the images shown that all parts were active at different levels while performing different functions. From these images, it was concluded that all parts are involved in assigned tasks or specific jobs, even if they are not active at the same time. In the above-mentioned data, the functions of different parts are included with their particular names. In one study, a generic brain activation map was constructed which gave a complete view of brain functioning (Brammer et al. 1997). According to this study, we can say that the myth has completely lost its charm.
Even surgeons have also operated on different parts of the brain, such as using electrical stimulation to treat epilepsy. Many other similar surgeries have been carried out, and all of them reveal that any damage to the brain can cause severe effects on human life and that there is no chance that humans use only 10 per cent of their brain. Even during surgeries, surgeons make sure to protect the brain, because this damage can lead to drastic results like loss of speech, memory, or even writing However, it has been proven that there is no evidence that nothing happens when you cut or remove a part of the brain (Aamodt and Wang 2008). The brain still has mysteries, but all we know is that it (all parts) works, not just 10 per cent. In recent years, scientists have discovered that the brain is more flexible than we thought, creating new ways to adapt to new tasks or recover from injury. There may not be huge reservoirs that do not exist in our brains, but what they do is remarkable.
In 2014, a film Lucy was released, which was about the use of 100 % human brain, the production cost of the film was 39 million dollars and the profit gained from the film was 463 million dollars and it was remained a hit for the whole year. Because it was an answer to the untold question of people that what will happen if we use our 100 per cent brain rather than 10 per cent. So, the myth is a profitable lie and a satisfactory answer for unsuccessful people in the world (Hickok 2014).
5.6. Misconception about the Myth
This myth is not only false, but also misunderstood by researchers and people. Misconceptions prolong the life of the myth. In the early years, the myth claimed that humans used only a small part of the brain, which later turned into 10 per cent of the brain. Then, another shade emerged that 10 per cent means is only the brain, which was misunderstood and interchanged to 10-percent use (Radford 1999). Another variation, developed by Craig Large, is that the human brain is divided into two parts, one is conscious and the other one is unconscious, the conscious part is about 10–20 % in use while the remaining 80–90 % is unconscious and unused (Schrempp and Craig 1991). So, the reason for the longevity of the myth is when one assumption is proven to be wrong or incorrect, believers shift the reason from one to another to prove the myth right. This myth is still accepted by many people because it gives individuals the most powerful and luring idea that we have psychic powers that can be achieved by unlocking our 90 per cent brain through focus and concentration.
5.7. An Alternative Approach to the Myth
There are two other facts that can be misleading. Nine out of ten cells in the brain are so-called glial cells (Sidman and Rakic 1973). These are the white matter, the supporting cells that provide physical and nutritional assistance to the other 10 per cent of the cells that make up the grey matter rather than the mind, namely the neurons. So maybe people hear that only 10 per cent of cells are transplanted hard and they assume that we can use glial cells as well. But these are completely different types of cells. They cannot suddenly transform into neurons and give us extra brain power. However, there is a very rare group of patients whose brain scans show something extraordinary. Bozek et al. reported that the patients with hydrocephalus had almost no brain tissue, but were still functioning (Bozek et al. 2014). Of course, this does not mean that the rest of us overuse our brains, it just means that these people are adapting to specific circumstances.
Of course, we can learn new things if we pay attention, and there is growing evidence in the field of neuroplasticity that this changes our brains. But we cannot access new parts of the brain. We make new connections between nerve cells or lose old connections that we no longer need. What we find most interesting about this myth is how disappointed people are when you tell them it is not true. Perhaps the 10-percent figure is so attractive because it is so low that it offers a huge potential for improvement. We all want to be better. We can do better if we try. Unfortunately, finding unused parts of our brain is not what it should be.
6. RECOMMENDATIONS
The article is all about the evidence to reject the 10-percent myth for the brain. But if we accept this myth, or say that it may be true to some extent, then there is a way to get it. This possibility is genomic variability, which is how individual neurons start to perform different functions. Even individuals can get diseases because of defective neurons that are also caused by genomic changes. Many studies have revealed that when Neanderthals and the ancestors of modern humans roamed the world half a million years ago, parts of their brains suddenly improved due to a major genetic change (Gregory et al. 2021). These mutations resulted in an increased number of brain cells; it could be a cognitive advantage for humans over their Neanderthal cousins who preceded modern humans. In 2014, the genome of Neanderthal was completely sequenced; the results stated that Neanderthals specifically have 96 amino acids that differ from modern humans, along with some other genetic changes (Prüfer et al. 2014).
Recently, a comparative study has been conducted comparing the TKTL1 gene, which encodes a protein in the early stages of fetal brain development, in humans and Neanderthals. The mutation has been detected in the human version of TKTL1 with a change in one amino acid, resulting in a different protein to that found in human ancestors. So, the researchers suggest that this protein may increase the proliferation of neural progenitor cells, which in particular increases the size of the neocortex by maturing more neurons as the brain develops. This is one of the reasons why modern humans gain cognitive function. The fossil record shows that humans and Neanderthals had roughly the same brain size (Weber 2023). This means that in modern humans the neocortex is either denser or takes up a larger proportion of the brain. It is surprising that such a minute change could have such a dramatic effect on mental ability. So, we can assume that there are chances that such kind of changes in DNA can enhance or even develop cognitive abilities in humans, such as observation skills, problem-solving behavior, and faster adaptations.
7. CONCLUSION
The human brain is a complex, dynamic, and highly functional organ. Each and every function in the human body, from emotional to physical, is under the control of the brain. The development of the human brain is a critical process because it consists of many stages, such as the embryonic level to the kindergarten level. According to the theory of evolution, humans evolved from the apes of Europe. The early animal evolution suggested that the nervous system of apes consisted of an autonomic and a somatic nervous system to process information and control body movements respectively. But over time humans achieved domain specification and maturation in the nervous system, due to which they gained sensory, language, and then social cognitive functions, also called higher cognitive functions. It is estimated that the human brain is made up of 100,000 million neurons which are connected to each other from different parts of the brain. Each part of the brain is involved in different functions of the body. In the early 1900s, a myth circulated that the average person used only 10 per cent of their brain. At that time, this myth was accepted by many prominent personalities, but with advances in technologies and innovations in the field of biology, the myth of the ten percent of the brain was rejected. The human brain has a highly organized structure and each function and its related part is directly or indirectly linked to the other function and part. Damage to any part of the brain can severely affect a person's survival. A widespread myth was an argument that if people reached 100 % brain capacity, then they would be able to have superpowers. But this argument is based on fallacy and ignorance. There are several reasons for this fallacy, such as Position emission tomography or PET scan and fMRI are clear proofs that almost all parts of the brain are connected to each other and all parts are functionally active. Although the brain uses certain parts for minor functions for a period of time, this does not mean that the brain is less active. For example, when we walk, we use some muscles of our legs to do it, not all the muscles work at the same time just to move our legs. Our brain works in a similar manner. According to the myth, the brain has ‘a used part’ in small proportions, while the ‘unused part’ contains a large area. If the parts are unnecessary and unused, they must be removed or disappear, just like the tails of humans. Now is the time to put the myth to rest, even though it has been survived for a whole century. If someone repeats the myth now, just ask them, ‘Really? Could you tell me what part we use in 10 per cent?’
Availability of Data and Materials
All the data is available in password protected laptop of M.M and will be available on request.
REFERENCES
Aamodt, S., and Wang, S. 2008. Tighten Your Belt, Strengthen Your Mind. New York Times, April 2. URL: https://www.nytimes.com/2008/04/02/opinion/02aamodt.html.
Achiron, R., and Achiron, A. 2001. Development of the Human Fetal Corpus Callosum: A High-Resolution, Cross-Sectional Sonographic Study. Ultrasound in Obstetrics and Gynecology 18 (4). https://doi.org/10.1046/j.0960-7692.2001.00512.x
Anderson, M., and Della Sala, S. 2012. Neuroscience in Education: An (opinionated) Introduction. In Della Sala, S., and Anderson, M. (eds.), Neuroscience in Education: The Good, the Bad, and the Ugly. Oxford: Oxford Academic. https://doi.org/10.1093/acprof:oso/9780199600496.003.0012.
Arora, M. 2020. The ‘Ten-Percent Brain Myth’ Guided with the Fundamentals of Jaina's Theory of Knowledge. International Journal of Psychosocial Rehabilitation 24 (08): 5977–5982.
Awang, J., Ramli, A. F., and Rahman, Z. A. 2021. Muslim Views on Other Religions: With Special Reference to Buddhism. HTS Teologiese Studies / Theological Studies 77 (4). https://doi.org/10.4102/hts.v77i4.6608.
Bae, B. Il, Jayaraman, D., and Walsh, C. A. 2015. Genetic Changes Shaping the Human Brain. Developmental Cell 32 (4). https://doi.org/10.1016/j.devcel.2015.01.035.
Barres, B. A. 2008. The Mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron 60 (3). https://doi.org/10.1016/j.neuron.2008.10.013.
Baum, G. L., Cui, Z., Roalf, D. R., Ciric, R., Betzel, R. F., Larsen, B., Cieslak, M., Cook, P. A., Xia, C. H., Moore, T. M., Ruparel, K., Oathes, D. J., Alexander-Bloch, A. F., Shinohara, R. T., Raznahan, A., Gur, R. E., Gur, R. C., Bassett, D. S., and Satterthwaite, T. D. 2020. Development of Structure–Function Coupling in Human Brain Networks during Youth. Proceedings of the National Academy of Sciences of the United States of America 117 (1). https://doi.org/10.1073/pnas.1912034117.
Bayly, P. V., Okamoto, R. J., Xu, G., Shi, Y., and Taber, L. A. 2013. A Cortical Folding Model Incorporating Stress-Dependent Growth Explains Gyral Wavelengths and Stress Patterns in the Developing Brain. Physical Biology 10 (1). https://doi.org/10.1088/1478-3975/10/1/016005.
Beyerstein, B. L. 1987. Neuroscience and Psi-ence. Behavioral and Brain Sciences 10 (04). https://doi.org/10.1017/s0140525x00054546.
Bozek, K., Wei, Y., Yan, Z., Liu, X., Xiong, J., Sugimoto, M., Tomita, M., Pääbo, S., Pieszek, R., Sherwood, C. C., Hof, P. R., Ely, J. J., Steinhauser, D., Willmitzer, L., Bangsbo, J., Hansson, O., Call, J., Giavalisco, P., and Khaitovich, P. 2014. Exceptional Evolutionary Divergence of Human Muscle and Brain Metabolomes Parallels Human Cognitive and Physical Uniqueness. PLoS Biology 12 (5). https://doi.org/10.1371/journal.pbio.1001871.
Brammer, M. J., Bullmore, E. T., Simmons, A., Williams, S. C. R., Grasby, P. M., Howard, R. J., Woodruff, P. W. R., and Rabe-Hesketh, S. 1997. Generic Brain Activation Mapping in Functional Magnetic Resonance Imaging: A Nonparametric Approach. Magnetic Resonance Imaging 15 (7). https:// doi.org/10.1016/S0730-725X(97)00135-5.
Bretas, R. V., Yamazaki, Y., and Iriki, A. 2020. Phase Transitions of Brain Evolution that Produced Human Language and Beyond. Neuroscience Research 161. https://doi.org/10.1016/j.neures.2019.11.010.
Budday, S., Nay, R., de Rooij, R., Steinmann, P., Wyrobek, T., Ovaert, T. C., and Kuhl, E. 2015. Mechanical Properties of Gray and White Matter Brain Tissue by Indentation. Journal of the Mechanical Behavior of Biomedical Materials 46. https://doi.org/10.1016/j.jmbbm.2015.02.024.
Budday, S., Steinmann, P., and Kuhl, E. 2015. Physical Biology of Human Brain Development. Frontiers in Cellular Neuroscience 9, July. https:// doi.org/10.3389/fncel.2015.00257.
Bystron, I., Blakemore, C., and Rakic, P. 2008. Development of the Human Cerebral Cortex: Boulder Committee Revisited. Nature Reviews Neuroscience 9 (2). https://doi.org/10.1038/nrn2252.
Carney, R. S. E., Bystron, I., López-Bendito, G., and Molnár, Z. 2007. Comparative Analysis of Extra-Ventricular Mitoses at Early Stages of Cortical Development in Rat and Human. Brain Structure and Function 212 (1). https://doi.org/10.1007/s00429-007-0142-4.
Cassirer, E. 2021. An Essay on Man: An Introduction to a Philosophy of Human Culture. Yale University Press.
Crispo, E. 2007. The Baldwin Effect and Genetic Assimilation: Revisiting Two Mechanisms of Evolutionary Change Mediated by Phenotypic Plasticity. Evolution 61 (11). https://doi.org/10.1111/j.1558-5646.2007.00203.x.
Dediu, D., and Ladd, D. R. 2007. Linguistic Tone is Related to the Population Frequency of the Adaptive Haplogroups of Two Brain Size Genes, ASPM and Microcephalin. Proceedings of the National Academy of Sciences of the United States of America, 104 (26). https://doi.org/10.1073/pnas.0610848104.
Enard, W. 2016. The Molecular Basis of Human Brain Evolution. Current Biology 26 (20). https://doi.org/10.1016/j.cub.2016.09.030.
Estrada, A., Garber, P. A., Rylands, A. B., Roos, Ch., Fernandez-Duque, E., Di, A., Nekaris, K. A.-I., Nijman, V., Heymann, E. W. et al. 2017. Impending Extinction Crisis of the World's Primates: Why Primates Matter. Science Advances, 3(e1600946).
Evans, P. D., Gilbert, S. L., Mekel-Bobrov, N., Vallender, E. J., Anderson, J. R., Vaez-Azizi, L. M., Tishkoff, S. A., Hudson, R. R., and Lahn, B. T. 2005. Microcephalin, a Gene Regulating Brain Size, Continues to Evolve Adaptively in Humans. Science 309 (5741). https://doi.org/10.1126/science.1113722.
Flourens, P. 1842. Recherches expérimentales sur les propriétés et les fonctions du système nerveux dans les animaux vertébrés. J.-B. Baillière.
Foote, A. D., Vijay, N., Ávila-Arcos, M. C., Baird, R. W., Durban, J. W., Fumagalli, M., Gibbs, R. A., Hanson, M. B., Korneliussen, T. S., Martin, M. D., Robertson, K. M., Sousa, V. C., Vieira, F. G., Vinar, T., Wade, P., Worley, K. C., Excoffier, L., Morin, P. A., Gilbert, M. T. P., and Wolf, J. B. W. 2016. Genome-Culture Coevolution Promotes Rapid Divergence of Killer Whale Ecotypes. Nature Communications 7. https://doi.org/10.1038/ncomms11693.
Franchini, L. F., and Pollard, K. S. 2015. Can a Few Non-Coding Mutations Make a Human Brain? BioEssays 37 (10). https://doi.org/10.1002/bies.201500049.
Funk, C. M., and Gazzaniga, M. S. 2009. The Functional Brain Architecture of Human Morality. Current Opinion in Neurobiology 19 (6). https:// doi.org/10.1016/j.conb.2009.09.011.
Grabowski, M. 2016. Bigger Brains Led to Bigger Bodies?: The Correlated Evolution of Human Brain and Body Size. Current Anthropology 57 (2). https://doi.org/10.1086/685655.
Gregory, M. D., Kippenhan, J. S., Kohn, P., Eisenberg, D. P., Callicott, J. H., Kolachana, B. and Berman, K. F. 2021. Neanderthal-Derived Genetic Variation is Associated with Functional Connectivity in the Brains of Living Humans. Brain Connectivity 11 (1): 38–44.
Haynes, R. L., Borenstein, N. S., Desilva, T. H., Folkerth, R. D., Liu, L. G., Volpe, J. J., and Kinney, H. C. 2005. Axonal Development in the Cerebral White Matter of the Human Fetus and Infant. Journal of Comparative Neurology 484 (2). https://doi.org/10.1002/cne.20453.
Herculano-Houzel, S. 2009. The Human Brain in Numbers: A Linearly Scaled-up Primate Brain. Frontiers in Human Neuroscience 3, November. Frontiers Media S. A. https://doi.org/10.3389/neuro.09.031.2009.
Hickok, G. 2014. Three Myths about the Brain. The New York Times, 3.
Holland, M. A., Miller, K. E., and Kuhl, E. 2015. Emerging Brain Morphologies from Axonal Elongation. Annals of Biomedical Engineering 43 (7). https://doi.org/10.1007/s10439-015-1312-9.
Hrvoj-Mihic, B., Bienvenu, T., Stefanacci, L., Muotri, A. R., and Semendeferi, K. 2013. Evolution, Development, and Plasticity of the Human Brain: From Molecules to Bones. Frontiers in Human Neuroscience, October. https://doi.org/10.3389/fnhum.2013.00707.
Iriki, A., and Taoka, M. 2012. Triadic (Ecological, Neural, and Cognitive) Niche Construction: A Scenario of Human Brain Evolution Extrapolating Tool Use and Language from the Control of reaching actions. Philosophical Transactions of the Royal Society B: Biological Sciences 367 (1585). https://doi.org/10.1098/rstb.2011.0190.
Kaas, J. H. 1997. Topographic Maps are Fundamental to Sensory Processing. Brain Research Bulletin 44 (2). https://doi.org/10.1016/S0361-9230(97)00094-4.
Kaminski, J., Call, J., and Tomasello, M. 2008. Chimpanzees Know What Others Know, but not What They Believe. Cognition 109 (2). https://doi.org/10.1016/j.cognition.2008.08.010.
Kostović, I., Judaš, M., Radoš, M., and Hrabač, P. 2002. Laminar Organization of the Human Fetal Cerebrum Revealed by Histochemical Markers and Magnetic Resonance Imaging. Cerebral Cortex 12 (5). https://doi.org/10.1093/cercor/12.5.536.
Laland, K. N., Odling-Smee, J., and Myles, S. 2010. How Culture Shaped the Human Genome: Bringing Genetics and the Human Sciences Together. Nature Reviews Genetics 11 (2). https://doi.org/10.1038/nrg2734.
Luders, E., Thompson, P. M., and Toga, A. W. 2010. The Development of the Corpus Callosum in the Healthy Human Brain. Journal of Neuroscience 30 (33). https://doi.org/10.1523/JNEUROSCI.5122-09.2010.
Marín-Padilla, M. 2012. The Human Brain Intracerebral Microvascular System: Development and Structure. Frontiers in Neuroanatomy, September. https://doi.org/10.3389/fnana.2012.00038.
Mekel-Bobrov, N., Gilbert, S. L., Evans, P. D., Vallender, E. J., Anderson, J. R., Hudson, R. R., Tishkoff, S. A., and Lahn, B. T. 2005. Ongoing Adaptive Evolution of ASPM, a Brain Size Determinant in Homo sapiens. Science 309 (5741). https://doi.org/10.1126/science.1116815.
Misson, J., Takahashi, T., and Caviness, V. S. 1991. Ontogeny of Radial and Other Astroglial Cells in Murine Cerebral Cortex. Glia 4 (2): 138–148. https://doi.org/10.1002/glia.440040205.
Mitchison, G. 1991. Neuronal Branching Patterns and the Economy of Cortical Wiring. Proceedings of the Royal Society B: Biological Sciences 245 (1313). https://doi.org/10.1098/rspb.1991.0102.
Mizutani, K. I., Yoon, K., Dang, L., Tokunaga, A., and Gaiano, N. 2007. Differential Notch Signalling Distinguishes Neural Stem Cells from Intermediate Progenitors. Nature 449 (7160). https://doi.org/10.1038/nature06090.
Molnár, Z., Adams, R., and Blakemore, C. 1998. Mechanisms Underlying the Early Establishment of Thalamocortical Connections in the rat. Journal of Neuroscience 18 (15). https://doi.org/10.1523/jneurosci.18-15-05723.
Muraskin, J., Dodhia, S., Lieberman, G., Garcia, J. O., Verstynen, T., Vettel, J. M., Sherwin, J., and Sajda, P. 2016. Brain Dynamics of Post-Task Resting State are Influenced by Expertise: Insights from Baseball Players. Human Brain Mapping 37 (12). https://doi.org/10.1002/hbm.23321.
O’Rahilly, R., and Müller, F. 2005. The Embryonic Human Brain: An Atlas of Developmental Stages, Third Edition. In The Embryonic Human Brain: An Atlas of Developmental Stages. 3rd ed. https://doi.org/10.1002/0471973084.
O’Toole, M., Lamoureux, P., and Miller, K. E. 2015. Measurement of Subcellular Force Generation in Neurons. Biophysical Journal 108 (5). https://doi.org/10.1016/j.bpj.2015.01.021.
Pakkenberg, B., and Gundersen, H. J. G. 1997. Neocortical Neuron Number in Humans: Effect of Sex and Age. Journal of Comparative Neurology, 384 (2). https://doi.org/10.1002/(SICI)1096-9861(19970728)384:2<312::
ID-CNE10>3.0.CO;2-K.
Pakkenberg, B., Pelvig, D., Marner, L., Bundgaard, M. J., Gundersen, H. J. G., Nyengaard, J. R., and Regeur, L. 2003. Aging and the Human Neocortex. Experimental Gerontology 38 (1–2). https://doi.org/10.1016/S0531-5565(02)00151-1.
Palmer, E. E., and Mowat, D. 2014. Agenesis of the Corpus Callosum: A Clinical Approach to Diagnosis. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics 166 (2). https://doi.org/10.1002/ajmg.c.31405.
Petrides, M. 1999. Dorsolateral Prefrontal Cortex: Comparative Cytoarchitectonic Analysis in the Human and the Macaque Brain and Corticocortical Connection Patterns. European Journal of Neuroscience, 11 (3). https:// doi.org/10.1046/j.1460-9568.1999.00518.x.
Premack, D. 2007. Human and Animal Cognition: Continuity and Discontinuity. In Proceedings of the National Academy of Sciences of the United States of America 104 (35). https://doi.org/10.1073/pnas.0706147104.
Prüfer, K., Racimo, F., Patterson, N., Jay, F., Sankararaman, S., Sawyer, S., Heinze, A., Renaud, G., Sudmant, P.H., De Filippo, C. and Li, H., 2014. The Complete Genome Sequence of a Neanderthal from the Altai Mountains. Nature 505 (7481): 43–49.
Radford, B. 1999. The Ten-Percent Myth. The Skeptical Inquirer 23: 52–53.
Rakic, S., and Zecevic, N. 2003. Emerging Complexity of Layer I in Human Cerebral Cortex. Cerebral Cortex 13 (10). https://doi.org/10.1093/cercor/13.10.1072.
Raybaud, C., Ahmad, T., Rastegar, N., Shroff, M., and Al Nassar, M. 2013. The Premature Brain: Developmental and Lesional Anatomy. Neuroradiology 55. https://doi.org/10.1007/s00234-013-1231-0.
Richman, D. P., Stewart, R. M., Hutchinson, J., and Caviness Jr, V. S. 1975. Mechanical Model of Brain Convolutional Development: Pathologic and Experimental Data Suggest a Model Based on Differential Growth within the Cerebral Cortex. Science 189 (4196).
Roffet-Salque, M., Marciniak, A., Valdes, P. J., Pawłowska, K., Pyzel, J., Czerniak, L., Krüger, M., Neil Roberts, C., Pitter, S., and Evershed, R. P. 2018. Evidence for the impact of the 8.2-kyBP climate event on Near Eastern early farmers. Proceedings of the National Academy of Sciences of the United States of America 115 (35). https://doi.org/10.1073/pnas.1803607115.
Ronan, L., Voets, N., Rua, C., Alexander-Bloch, A., Hough, M., Mackay, C., Crow, T. J., James, A., Giedd, J. N., and Fletcher, P. C. 2014. Differential Tangential Expansion as a Mechanism for Cortical Gyrification. Cerebral Cortex 24 (8). https://doi.org/10.1093/cercor/bht082.
Schrempp, G., and Craig, R. D. 1991. Dictionary of Polynesian Mythology. The Journal of American Folklore 104 (412). https://doi.org/10.2307/541247.
Sherwood, C. C., Bauernfeind, A. L., Bianchi, S., Raghanti, M. A., and Hof, P. R. 2012. Human Brain Evolution Writ Large and Small. Progress in Brain Research 195. https://doi.org/10.1016/B978-0-444-53860-4.00011-8.
Sidman, R. L., and Rakic, P. 1973. Neuronal Migration, with Special Reference to Developing Human Brain: A Review. Brain Research 62 (1). https://doi.org/10.1016/0006-8993(73)90617-3.
Snell-Rood, E. C., and Wick, N. 2013. Anthropogenic Environments Exert Variable Selection on Cranial Capacity in Mammals. Proceedings of the Royal Society B: Biological Sciences 280 (1769). https://doi.org/10.1098/rspb.2013.1384.
Sun, T., and Hevner, R. F. 2014. Growth and Folding of the Mammalian Cerebral Cortex: From Molecules to Malformations. Nature Reviews Neuroscience 15 (4). https://doi.org/10.1038/nrn3707.
Suter, D. M., and Miller, K. E. 2011. The Emerging Role of Forces in Axonal Elongation. Progress in Neurobiology 94 (2). https://doi.org/10.1016/j.pneurobio.2011.04.002.
Takahashi, E., Folkerth, R. D., Galaburda, A. M., and Grant, P. E. 2012. Emerging Cerebral Connectivity in the Human Fetal Brain: An MR Tractography Study. Cerebral Cortex 22 (2). https://doi.org/10.1093/cercor/bhr126.
van Dijk, W., and Lane, H. B. 2020. The Brain and the US Education System: Perpetuation of Neuromyths. Exceptionality 28 (1). https://doi.org/10.1080/09362835.2018.1480954.
Van Essen, D. C., and Drury, H. A. 1997. Structural and Functional Analyses of Human Cerebral Cortex Using a Surface-Based Atlas. Journal of Neuroscience 17 (18). https://doi.org/10.1523/jneurosci.17-18-07079.1997.
Ventura-Antunes, L., Mota, B., and Herculano-Houzel, S. 2013. Different Scaling of White Matter Volume, Cortical Connectivity, and Gyrification across Rodent and Primate Brains. Frontiers in Neuroanatomy, March. https://doi.org/10.3389/fnana.2013.00003.
Watson, A. M., Rose, A. H., Gibson, G. A., Gardner, C. L., Sun, C., Reed, D. S., Metthew Lam, L. K., Croix, C. M. S., Strick, P. L., Klimstra, W. B., and Watkins, S. C. 2017. Ribbon Scanning Confocal for High-Speed High-Resolution Volume Imaging of Brain. PLoS ONE, 12 (7). https://doi.org/10.1371/journal.pone.0180486.
Weber, G.W. 2023. Quantum Leaps in Human Biocultural Evolution and the Relationship to Cranial Capacity. Life 13 (4): 1030.
Xu, G., Knutsen, A. K., Dikranian, K., Kroenke, C. D., Bayly, P. V., and Taber, L. A. 2010. Axons Pull on the Brain, but Tension does not Drive Cortical Folding. Journal of Biomechanical Engineering 132 (7). https:// doi.org/10.1115/1.4001683.
Zecevic, N., Chen, Y., and Filipovic, R. 2005. Contributions of Cortical Subventricular Zone to the Development of the Human Cerebral Cortex. Journal of Comparative Neurology 491 (2). https://doi.org/10.1002/cne.20714.
Zilles, K., Palomero-Gallagher, N., and Amunts, K. 2013. Development of Cortical Folding during Evolution and Ontogeny. Trends in Neurosciences 36 (5). https://doi.org/10.1016/j.tins.2013.01.006.
